|
 |
 |
 |
 |
|
|
|






Disposition Models

Disposition is the term often used to integrate all the processes of distribution, biotransformation, and elimination. Disposition models have been derived to describe how a toxicant moves within the body with time (also known as kinetic models). The disposition models are named for the number of areas of the body (known as compartments) that the chemical may go to. For example, blood is a compartment. Fat (adipose) tissue, bone, liver, kidneys, and brain are other major compartments.
Kinetic models may be a one-compartment open model, a two-compartment open model, or a multiple compartment model. The one-compartment open model describes the disposition of a substance that is introduced and distributed instantaneously and evenly in the body, and eliminated at a rate and amount that is proportional to the amount left in the body. This is known as a "first-order" rate, and represented as the logarithm of concentration in blood as a linear function of time.
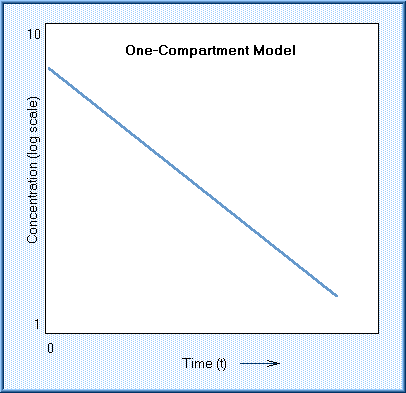
The half-life of the chemical that follows a one-compartment model is simply the time required for half the chemical to be lost from the plasma. Only a few chemicals actually follow the simple, first-order, one compartment model.
For most chemicals, it is necessary to describe the kinetics in terms of at least a two-compartment model. In the two-compartment open model, the chemical enters and distributes in the first compartment, which is normally blood. It is then distributed to another compartment from which it can be eliminated or it may return to the first compartment. Concentration in the first compartment declines smoothly with time. Concentration in the second compartment rises, peaks, and subsequently declines as the chemical is eliminated from the body.
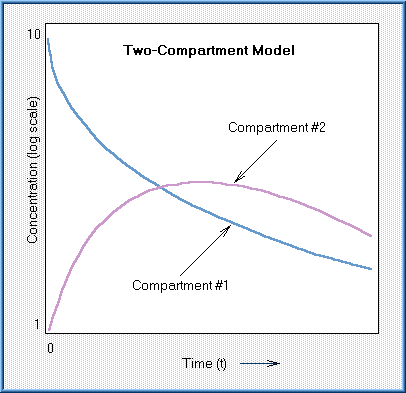
A half-life for a chemical whose kinetic behavior fits a two-compartment model is often referred to as the "biological half-life." This is the most commonly used measure of the kinetic behavior of a xenobiotic.
Frequently the kinetics of a chemical within the body can not be adequately described by either of these models since there may be several peripheral body compartments that the chemical may go to, including long-term storage. In addition, biotransformation and elimination of a chemical may not be simple processes but subject to different rates as the blood levels change.

  
|
|
|
|


