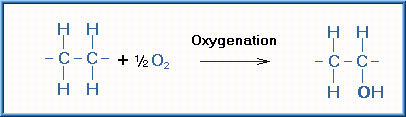|
 |
 |
 |
 |
|
|
|






Chemical Reactions

Chemical reactions are continually taking place in the body. They are a normal aspect of life, participating in the building up of new tissue, tearing down of old tissue, conversion of food to energy, disposal of waste materials, and elimination of toxic xenobiotics. Within the body is a magnificent assembly of chemical reactions, which is well orchestrated and called upon as needed. Most of these chemical reactions occur at significant rates only because specific proteins, known as enzymes, are present to catalyze them, that is, accelerate the reaction. A catalyst is a substance that can accelerate a chemical reaction of another substance without itself undergoing a permanent chemical change.
Enzymes are the catalysts for nearly all biochemical reactions in the body. Without these enzymes, essential biotransformation reactions would take place slowly or not at all, causing major health problems. An example is the inability of persons that have phenylketonuria (PKU) to use the artificial sweetener, aspartame (in Equal®). Aspartame is basically phenylalanine, a natural constituent of most protein-containing foods. Some persons are born with a genetic condition in which the enzyme that can biotransform phenylalanine to tyrosine (another amino acid), is defective. As the result, phenylalanine can build up in the body and cause severe mental retardation. Babies are routinely checked at birth for PKU. If they have PKU, they must be given a special diet to restrict the intake of phenylalanine in infancy and childhood.
These enzymatic reactions are not always simple biochemical reactions. Some enzymes require the presence of cofactors or co-enzymes in addition to the substrate (the substance to be catalyzed) before their catalytic activity can be exerted. These co-factors exist as a normal component in most cells and are frequently involved in common reactions to convert nutrients into energy (Vitamins are an example of co-factors). It is the drug or chemical transforming enzymes that hold the key to xenobiotic transformation. The relationship of substrate, enzyme, co-enzyme, and transformed product is illustrated below:
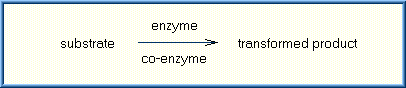
Most biotransforming enzymes are high molecular weight proteins, composed of chains of amino acids linked together by peptide bonds. A wide variety of biotransforming enzymes exist. Most enzymes will catalyze the reaction of only a few substrates, meaning that they have high "specificity". Specificity is a function of the enzyme's structure and its catalytic sites. While an enzyme may encounter many different chemicals, only those chemicals (substrates) that fit within the enzymes convoluted structure and spatial arrangement will be locked on and affected. This is sometimes referred to as the "lock and key" relationship. As shown in the diagram, when a substrate fits into the enzyme's structure, an enzyme-substrate complex can be formed. This allows the enzyme to react with the substrate with the result that two different products are formed. If the substrate does not fit into the enzyme, no complex will be formed and thus no reaction can occur.
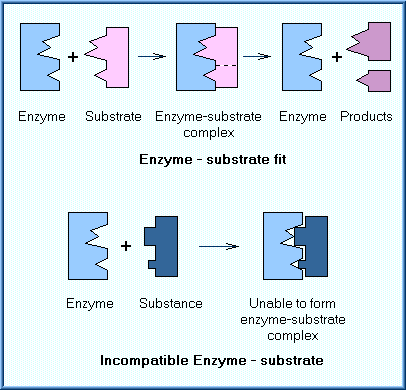
The array of enzymes range from those that have absolute specificity to those that have broad and overlapping specificity. In general, there are three main types of specificity:
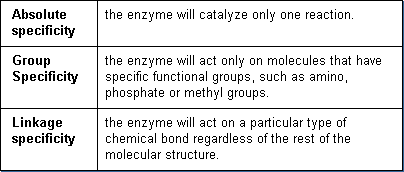
For example, formaldehyde dehydrogenase has absolute specificity since it catalyzes only the reaction for formaldehyde. Acetylcholinesterase has absolute specificity for biotransforming the neurotransmitting chemical, acetylcholine. Alcohol dehydrogenase has group specificity since it can biotransform several different alcohols, including methanol and ethanol. N-oxidation can catalyze a reaction of a nitrogen bond, replacing the nitrogen with oxygen.
The names assigned to enzymes may seem confusing at first. However, except for some of the originally studied enzymes (such as pepsin and trypsin), a convention has been adopted to name enzymes. Enzyme names end in "ase" and usually combine the substrate acted on and the type of reaction catalyzed. For example, alcohol dehydrogenase is an enzyme that biotransforms alcohols by the removal of a hydrogen. The result is a completely different chemical, an aldehyde or ketone.
The biotransformation of ethyl alcohol to acetaldehyde is depicted below:
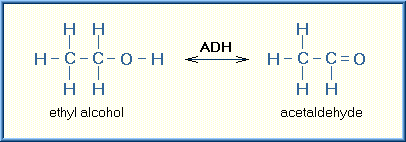
ADH = alcohol dehydrogenase, a specific catalyzing enzyme
NAD = nicotinamide adenine dinucleotide, a common cellular reducing agent
By now you know that the transformation of a specific xenobiotic can be either beneficial or harmful, and perhaps both depending on the dose and circumstances. A good example is the biotransformation of acetaminophen (Tylenol®), a commonly used drug to reduce pain and fever. When the prescribed doses are taken, the desired therapeutic response is observed with little or no toxicity. However, when excessive doses of acetaminophen are taken, hepatotoxicity can occur. This is because acetaminophen normally undergoes rapid biotransformation with the metabolites quickly eliminated in the urine and feces.
At high doses, the normal level of enzymes may be depleted and the acetaminophen is available to undergo reaction by an additional biosynthetic pathway, which produces a reactive metabolite that is toxic to the liver. For this reason, a user of Tylenol® is warned not to take the prescribed dose more frequently than every 4-6 hours and not to consume more than four doses within a 24-hour period. Biotransforming enzymes, like most other biochemicals, are available in a normal amount and in some situations can be "used up" at a rate that exceeds the bodies ability to replenish them. This illustrates the frequently used phrase, the "Dose Makes the Poison."
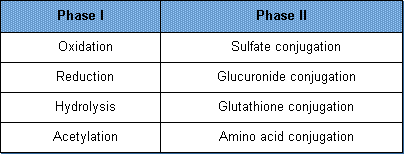
Biotransformation reactions are categorized not only by the nature of their reactions, e.g., oxidation, but also by the normal sequence with which they tend to react with a xenobiotic. They are usually classified as Phase I and Phase II reactions. Phase I reactions are generally reactions which modify the chemical by adding a functional structure. This allows the substance to "fit" into the Phase II enzyme so that it can become conjugated (joined together) with another substance. Phase II reactions consist of those enzymatic reactions that conjugate the modified xenobiotic with another substance. The conjugated products are larger molecules than the substrate and generally polar in nature (water-soluble). Thus, they can be readily excreted from the body. Conjugated compounds also have poor ability to cross cell membranes.
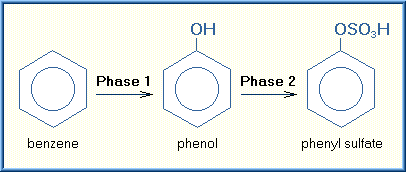
In some cases, the xenobiotic already has a functional group that can be conjugated and the xenobiotic can be biotransformed by a Phase II reaction without going through a Phase I reaction. A good example is phenol that can be directly conjugated into a metabolite that can then be excreted. The biotransformation of benzene requires both Phase I and Phase II reactions. As illustrated below, benzene is biotransformed initially to phenol by a Phase I reaction (oxidation). Phenol has the functional hydroxyl group that is then conjugated by a Phase II reaction (sulphation) to phenyl sulfate.
The major transformation reactions for xenobiotics are listed below:
Phase I Reactions

Phase I biotransformation reactions are simple reactions as compared to Phase II reactions. In Phase I reactions, a small polar group (containing both positive and negative charges) is either exposed on the toxicant or added to the toxicant. The three main Phase I reactions are oxidation, reduction, and hydrolysis.
Oxidation is a chemical reaction in which a substrate loses electrons. There are a number of reactions that can achieve the removal of electrons from the substrate. Addition of oxygen was the first of these reactions discovered and thus the reaction was named oxidation. However, many of the oxidizing reactions do not involve oxygen. The simplest type of oxidation reaction is dehydrogenation, that is the removal of hydrogen from the molecule. Another example of oxidation is electron transfer that consists simply of the transfer of an electron from the substrate.
Examples of these types of oxidizing reactions are illustrated below:
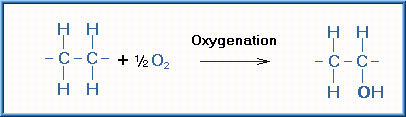
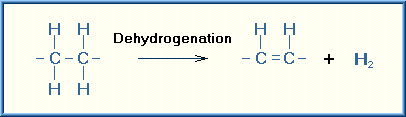
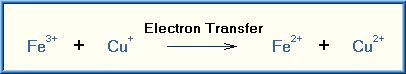
The specific oxidizing reactions and oxidizing enzymes are numerous and several textbooks are devoted to this subject. Most of the reactions are self-evident from the name of the reaction or enzyme involved. Listed are several of these oxidizing reactions.
 |
 |
 |
|  | alcohol dehydrogenation
|
 |
|  | aldehyde dehydrogenation
|
 |
|  | alkyl/acyclic hydroxylation
|
 |
|  | aromatic hydroxylation
|
 |
|  | deamination
|
 |
|  | desulfuration
|
 |
|  | N-dealkylation
|
 |
|  | N-hydroxylation
|
 |
|  | N-oxidation
|
 |
|  | O-dealkylation
|
 |
|  | sulphoxidation
|
 |
Reduction is a chemical reaction in which the substrate gains electrons. Reductions are most likely to occur with xenobiotics in which oxygen content is low. Reductions can occur across nitrogen-nitrogen double bonds (azo reduction) or on nitro groups (NO2). Frequently, the resulting amino compounds are oxidized forming toxic metabolites. Some chemicals such as carbon tetrachloride can be reduced to free radicals, which are quite reactive with biological tissues. Thus, reduction reactions frequently result in activation of a xenobiotic rather than detoxification. An example of a reduction reaction in which the nitro group is reduced is illustrated below:
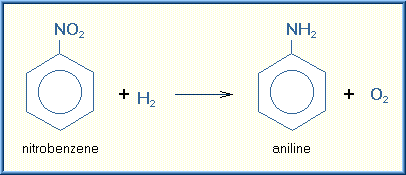
There are fewer specific reduction reactions than oxidizing reactions. The nature of these reactions is also self-evident from their name. Listed are several of the reducing reactions.
 |
 |
 |
|  | azo reduction
|
 |
|  | dehalogenation
|
 |
|  | disulfide reduction
|
 |
|  | nitro reduction
|
 |
|  | N-oxide reduction
|
 |
|  | sulfoxide reduction
|
 |
Hydrolysis is a chemical reaction in which the addition of water splits the toxicant into two fragments or smaller molecules. The hydroxyl group (OH-) is incorporated into one fragment and the hydrogen atom is incorporated into the other. Larger chemicals such as esters, amines, hydrazines, and carbamates are generally biotransformed by hydrolysis. The example of the biotransformation of procaine (local anesthetic) which is hydrolyzed to two smaller chemicals is illustrated below:
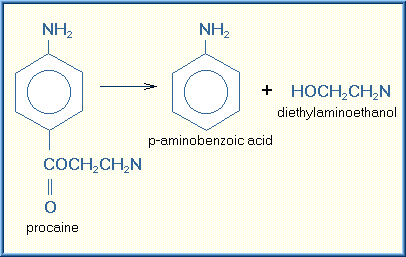
Toxicants that have undergone Phase I biotransformation are converted to metabolites that are sufficiently ionized, or hydrophilic, to be either eliminated from the body without further biotransformation or converted to an intermediate metabolite that is ready for Phase II biotransformation. The intermediates from Phase I transformations may be pharmacologically more effective and in many cases more toxic than the parent xenobiotic.
Phase II Reactions

A xenobiotic that has undergone a Phase I reaction is now a new intermediate metabolite that contains a reactive chemical group, e.g., hydroxyl (-OH), amino (-NH2), and carboxyl (-COOH). Many of these intermediate metabolites do not possess sufficient hydrophilicity to permit elimination from the body. These metabolites must undergo additional biotransformation as a Phase II reaction.
Phase II reactions are conjugation reactions, that is, a molecule normally present in the body is added to the reactive site of the Phase I metabolite. The result is a conjugated metabolite that is more water-soluble than the original xenobiotic or Phase I metabolite. Usually the Phase II metabolite is quite hydrophilic and can be readily eliminated from the body. The primary Phase II reactions are:
 |
 |
 |
|  | glucuronide conjugation - most important reaction
|
 |
|  | sulfate conjugation - important reaction
|
 |
|  | acetylation
|
 |
|  | amino acid conjugation
|
 |
|  | glutathione conjugation
|
 |
|  | methylation
|
 |
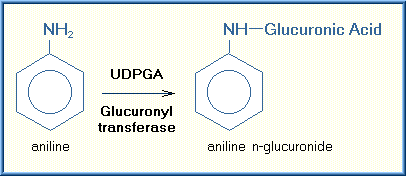
Glucuronide conjugation is one of the most important and common Phase II reactions. One of the most popular molecules added directly to the toxicant or its phase I metabolite is glucuronic acid, a molecule derived from glucose, a common carbohydrate (sugar) that is the primary source of energy for cells. The sites of glucuronidation reactions are substrates having an oxygen, nitrogen, or sulfur bond. This includes a wide array of xenobiotics as well as endogenous substances, such as bilirubin, steroid hormones and thyroid hormones. Glucuronidation is a high-capacity pathway for xenobiotic conjugation. Glucuronide conjugation usually decreases toxicity. Although there are some notable exceptions, for example, the production of carcinogenic substances. The glucuronide conjugates are generally quite hydrophilic and are excreted by the kidney or bile, depending on the size of the conjugate. The glucuronide conjugation of aniline is illustrated below:
Sulfate conjugation is another important Phase II reaction that occurs with many xenobiotics. In general, sulfation decreases the toxicity of xenobiotics. Unlike glucuronic acid conjugates that are often eliminated in the bile, the highly polar sulfate conjugates are readily secreted in the urine. In general, sulfation is a low-capacity pathway for xenobiotic conjugation. Often glucuronidation or sulfation can conjugate the same xenobiotics.

  
|
|
|
|