|
 |
 |
 |
 |
|
|
|






Clinical Investigations

Knowledge of toxicity of xenobiotics to humans is derived by three methods:
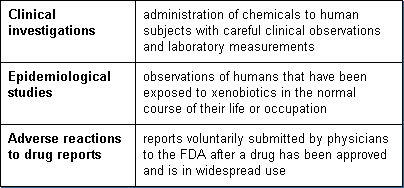
Clinical investigations are a component of the Investigational New Drug Applications (IND) submitted to FDA. Clinical investigations are conducted only after the non-clinical laboratory studies have been completed.
Toxicity studies using human subjects require strict ethical considerations. They are primarily conducted for new pharmaceutical applications submitted to FDA for approval.
Generally, toxicity found in animal studies occurs with similar incidence and severity in humans. Differences sometimes occur, thus clinical tests with humans are needed to confirm the results of non-clinical laboratory studies.
FDA clinical investigations are conducted in three phases. Phase 1 consists of testing the drug in a small group of 20-80 patients. Information obtained in Phase 1 studies is used to design Phase 2 studies. In particular to:
 |
 |
 |
|  | determine the drug's pharmacokinetics and pharmacological effects
|
 |
|  | elucidate its metabolism
|
 |
|  | study the mechanism of action of the drug
|
 |
Phase 2 studies are more extensive involving several hundred patients and are used to:
 |
 |
 |
|  | determine the short-term side effects of the drug
|
 |
|  | determine the risks associated with the drug
|
 |
|  | evaluate the effectiveness of the drug for treatment of a particular disease or condition
|
 |
Phase 3 studies are expanded controlled and uncontrolled trials conducted with several hundred to several thousand patients. They are designed to:
 |
 |
 |
|  | gather additional information about effectiveness and safety
|
 |
|  | evaluate overall benefit-risk relationship of the drug
|
 |
|  | provide the basis for the precautionary information that accompanies the drug
|
 |

  
|
|
|
|

