|
 |
 |
 |
 |
|
|
|






Animal Testing for Toxicity

Animal tests for toxicity are conducted prior to human clinical investigations as part of the non-clinical laboratory tests of pharmaceuticals. For pesticides and industrial chemicals, human testing is rarely conducted. Animal test results often represent the only means by which toxicity in humans can be effectively predicted.
With animal tests:
 |
 |
 |
|  | chemical exposure can be precisely controlled
|
 |
|  | environmental conditions can be well-controlled
|
 |
|  | virtually any type of toxic effect can be evaluated
|
 |
|  | the mechanism by which toxicity occurs can be studied
|
 |
Methods to evaluate toxicity exist for a wide variety of toxic effects. Some procedures for routine safety testing have been standardized. Standardized animal toxicity tests are highly effective in detecting toxicity that may occur in humans. Concern for animal welfare has resulted in tests that use humane procedures and only the number of animals needed for statistical reliability.
To be standardized, a test procedure must have scientific acceptance as the most meaningful assay for the toxic effect. Toxicity testing can be very specific for a particular effect, such as dermal irritation, or it may be general, such as testing for unknown chronic effects.
Standardized tests have been developed for the following effects:
 |
 |
 |
|  | Acute Toxicity
|
 |
|  | Subchronic Toxicity
|
 |
|  | Chronic Toxicity
|
 |
|  | Carcinogenicity
|
 |
|  | Reproductive Toxicity
|
 |
|  | Developmental Toxicity
|
 |
|  | Dermal Toxicity
|
 |
|  | Ocular Toxicity
|
 |
|  | Neurotoxicity
|
 |
|  | Genetic Toxicity
|
 |
Species selection varies with the toxicity test to be performed. There is no single species of animal that can be used for all toxicity tests. Different species may be needed to assess different types of toxicity. In some cases, it may not be possible to use the most desirable animal for testing because of animal welfare or cost considerations. For example, use of monkeys and dogs is restricted to special cases, even though they represent the species that may react closest to humans.
Rodents and rabbits are the most commonly used laboratory species due to their availability, low costs in breeding and housing, and past history in producing reliable results.
The toxicologist attempts to design an experiment to duplicate the potential exposure of humans as closely as possible. For example:
 |
 |
 |
|  | The route of exposure should simulate that of human exposure. Most standard tests use inhalation, oral, or dermal routes of exposure.
|
 |
|  | The age of test animals should relate to that of humans. Testing is normally conducted with young adults, although newborn or pregnant animals may be used in some cases.
|
 |
|  | For most routine tests, both sexes are used. Sex differences in toxic response are minimal, except for toxic substances with hormonal properties.
|
 |
|  | Dose levels are normally selected so as to determine the threshold as well as dose-response relationship. Usually, a minimum of three dose levels are used.
|
 |
Acute Toxicity

Acute toxicity tests are generally the first tests conducted. They provide data on the relative toxicity likely to arise from a single or brief exposure. Standardized tests are available for oral, dermal, and inhalation exposures. Basic parameters of these tests are:
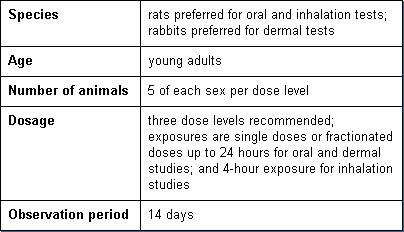
Subchronic Toxicity

Subchronic toxicity tests are employed to determine toxicity likely to arise from repeated exposures of several weeks to several months. Standardized tests are available for oral, dermal, and inhalation exposures. Detailed clinical observations and pathology examinations are conducted. Basic parameters of these tests are:
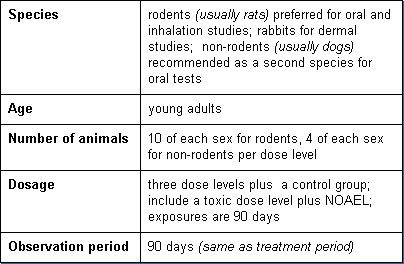
Chronic Toxicity

Chronic toxicity tests determine toxicity from exposure for a substantial portion of a subject's life. They are similar to the subchronic tests except that they extend over a longer period of time and involve larger groups of animals. Basic parameters of these tests include:
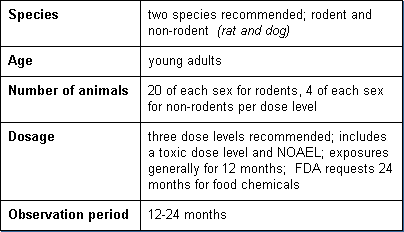
Carcinogenicity

Carcinogenicity tests are similar to chronic toxicity tests. However, they extend over a longer period of time and require larger groups of animals in order to assess the potential for cancer. Basic parameters of these tests are:
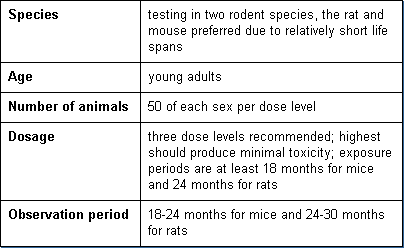
Reproductive Toxicity

Reproductive toxicity testing is intended to determine the effects of substances on gonadal function, conception, birth, and the growth and development of the offspring. The oral route is preferred. Basic parameters of these tests are:
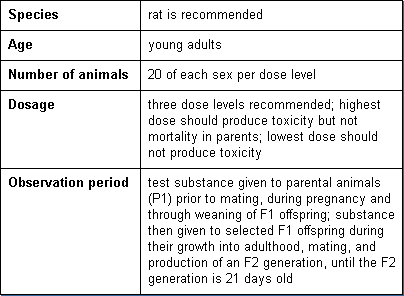
Developmental Toxicity

Developmental toxicity testing detects the potential for substances to produce embryotoxicity and birth defects. Basic parameters of this test are:
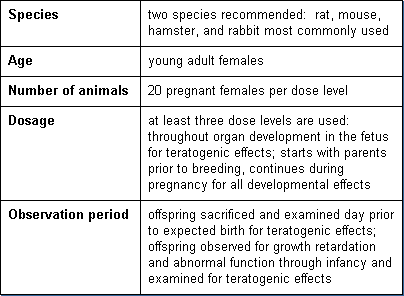
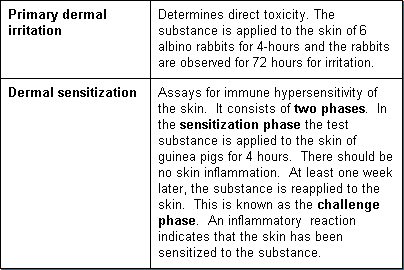
Dermal Toxicity

Dermal toxicity tests determine the potential for an agent to cause irritation and inflammation of the skin. This may be the result of direct damage to the skin cells by a substance. It may also be an indirect response due to sensitization from prior exposure. There are two dermal toxicity tests:
Ocular Toxicity

Ocular toxicity is determined by applying a test substance for one second to the eyes of 6 test animals, usually rabbits. The eyes are then carefully examined for 72-hours, using a magnifying instrument to detect minor effects. The ocular reaction may occur on the cornea, conjunctiva, or iris. It may be simple irritation that is reversible and quickly disappears or the irritation may be severe and produce corrosion, an irreversible condition.
The eye irritation test is commonly known as the "Draize Test." This test has been targeted by animal welfare groups as an inhumane procedure due to pain that may be induced in the eye. The test allows the use of an eye anesthetic in the event pain is evident. The Draize Test is a reliable predictor of human eye response. However, research to develop alternative testing procedures that do not use live animals is underway. While some cell and tissue assays are promising, they have not as yet proved as reliable as the animal test.
Neurotoxicity

A battery of standardized neurotoxicity tests has recently been developed to supplement the delayed neurotoxicity test in domestic chickens (hens). The hen assay determines delayed neurotoxicity resulting from exposure to anticholinergic substances, such as certain pesticides. The hens are protected from the immediate neurological effects of the test substance and observed for 21 days for delayed neurotoxicity. Other neurotoxicity tests include measurements of:
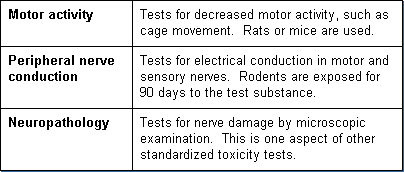
Genetic Toxicity

Genetic toxicity is determined using a wide range of test species including whole animals and plants (e.g., rodents, insects, and corn), microorganisms, and mammalian cells. A large variety of tests have been developed to measure gene mutations, chromosome changes, and DNA activity. The most common gene mutation tests involve:
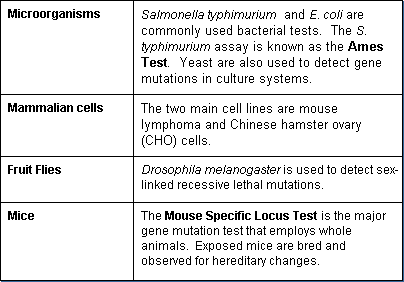
Chromosomal effects can be detected by a variety of tests, some involving whole animals (in vivo). Some use cell systems (in vitro). Several assays are available to test for chemically induced chromosome aberrations in whole animals. The most common tests are:
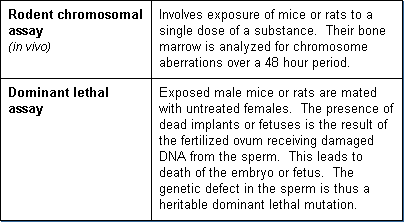
Additional in vivo chromosomal assays are:
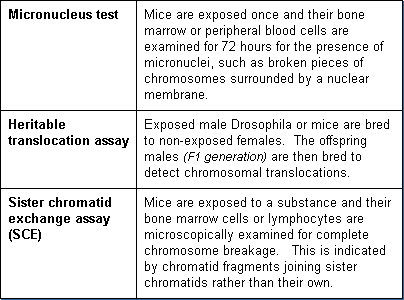
In vitro tests for chromosomal effects involve the exposure of cell cultures and microscopic examination for chromosome damage. The most commonly used cell lines are Chinese Hamster Ovary (CHO) cells and human lymphocyte cells. The CHO cells are easy to culture, grow rapidly, and have a low chromosome number (22) which makes for easier identification of chromosome damage.
Human lymphocytes are more difficult to culture. They are obtained from healthy human donors with known medical histories. The results of these assays are potentially more relevant to determine effects of xenobiotics which induce mutations in humans.
Two widely used genotoxicity tests measure DNA damage and repair which is not mutagenicity. DNA damage is considered the first step in the process of mutagenesis. The most commonly used test for unscheduled DNA synthesis (UDS) involves exposure of mammalian cells in culture to a test substance. UDS is measured by the uptake of tritium-labeled thymidine into the DNA of the cells. Rat hepatocytes or human fibroblasts are the common mammalian cell lines used.
Another assay to detect DNA damage involves the exposure of repair-deficient E. coli or B. subtilis. DNA damage can not be repaired so the cells die or their growth may be inhibited.

  
|
|
|
|











