|
 |
 |
 |
 |
|
|
|






Chemicals

Most toxic effects are initiated by chemical interactions in which a foreign chemical or physical agent interferes with or damages normal chemicals of the body. This results in the body chemical being unable to carry out its' function in maintaining homeostasis. There are many ways that this can happen, e.g., interference with absorption or disposition of an essential nutrient, interference with nerve transmission, or damage to a cell organelle preventing its' functioning. These mechanisms of xenobiotic toxicity will be discussed in a subsequent section.
Types of Physiological Chemicals

There are basically three categories of chemicals normally functioning in the body.
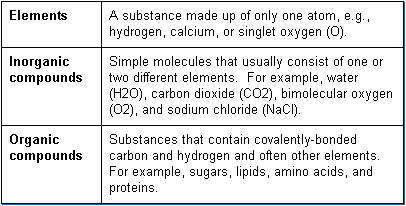
Elements are components of all chemical compounds. Of the 92 naturally occurring elements, only 20 are normally found in the body. Seven of these, carbon, oxygen, hydrogen, calcium, nitrogen, phosphorous, and sulfur make up approximately 99% of the human body weight. In most cases, the elements are components of inorganic or organic compounds. In a few cases, however, elements themselves may enter into chemical reactions in the body, e.g., oxygen during cell respiration, sodium in neurotransmission, and arsenic and lead in impaired mitochondrial metabolism.
Inorganic compounds are important in the body and responsible for many simple functions. The major inorganic compounds are water (H2O), bimolecular oxygen (O2), carbon dioxide (CO2), and some acids, bases, and salts. The body is composed of 60-75% water. Oxygen is required by all cells for cellular metabolism and circulating blood must be well oxygenated for maintenance of life. Carbon dioxide is a waste product of cells and must be eliminated or a serious change in pH can occur, known as acidosis. A balance in acids, bases, and salts must be maintained to assure homeostasis of blood pH and electrolyte balance.
Organic compounds are involved in nearly all biochemical activities involved in normal cellular metabolism and function. The mechanisms by which xenobiotics cause cellular and biochemical toxicity are predominantly related to changes to organic compounds. The main feature that differentiates organic compounds from inorganic compounds is that organic compounds always contain carbon. Most organic compounds are also relatively large molecules. There are five major categories of organic compounds involved in normal physiology of the body, namely carbohydrates, lipids, proteins, nucleic acids, and high-energy compounds.
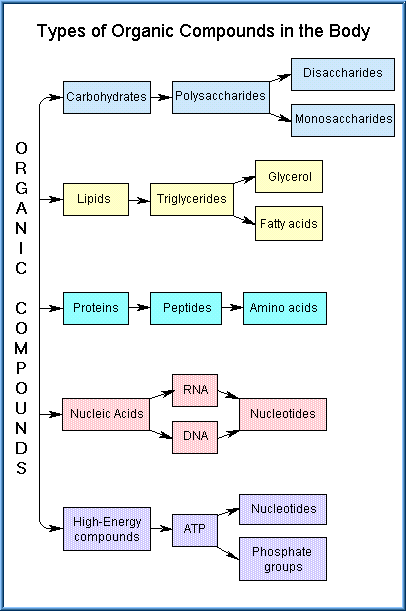
Most carbohydrates serve as sources of energy for the body. They are converted to glucose, which in turn is used by the cells in cell respiration. Other carbohydrates become incorporated as structural components of genetic macromolecules. For example, deoxyribose is part of DNA (the genetic material of chromosomes) and ribose is part of RNA (which regulates protein synthesis).
Lipids are essential substances of all cells and serve as a major energy reserve. They may be stored as fatty acids or as triglycerides. Other types of lipids are the steroids and phospholipids. Cholesterol is a lipid that is a component of cell membranes and is utilized in the production of the sex hormones, e.g., testosterone and estrogen. Phospholipids serve as the main components of the phospholipid bilayer cell membrane.
The most diverse and abundant of organic compounds in the body is the group of proteins. There are about 100,000 different kinds of proteins, which account for about 20% of the body weight. The building blocks for proteins are the 20 amino acids, which contain carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur. Most protein molecules are large and consist of 50-1000 amino acids bonded together in a very precise structural arrangement. Even the slightest change in the protein molecule alters its function.
Proteins perform a large variety of important functions. Some proteins have a structural function such as the protein pores in cell membranes, keratin in skin and hair, collagen in ligaments and tendons, and myosin in muscles. Hemoglobin and albumen are proteins that carry oxygen and nutrients in the circulating blood. Antibodies and hormones are proteins. A particularly important group of proteins are the enzymes.
Enzymes, which are catalysts, are compounds that accelerate chemical reactions, without themselves being permanently changed. Each enzyme is quite specific in that it will catalyze only one type of reaction. Enzymes are quite vulnerable to damage by xenobiotics and many toxic reactions are manifested by enzyme denaturation (change in shape) or enzyme inhibition.
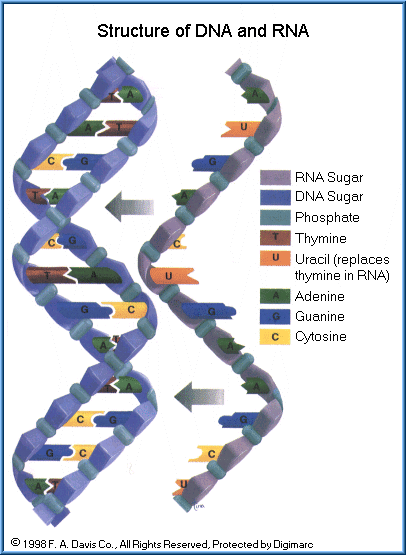
Nucleic acids are large organic compounds which store and process information at the molecular level inside virtually all body cells. Three types of nucleic acids are present, deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and adenosine triphosphate (ATP). Nucleic acids are very large molecules composed of smaller units known as nucleotides. A nucleotide consists of a pentose sugar, a phosphate group, and four nitrogenous bases. The sugar in DNA is deoxyribose while the bases are adenine, guanine, cytosine and thymine. RNA consists of the sugar, ribose and the four bases are adenine, guanine, cytosine and uracil. These two types of molecules are known as the molecules of life. For without them, cells could not reproduce and animal reproduction would not occur.
DNA is in the nucleus and makes up the chromosomes of cells. It is the genetic code for hereditary characteristics. RNA is located in the cytoplasm of cells and regulates protein synthesis, using information provided by the DNA. Some toxic agents can damage the DNA causing a mutation, which can lead to death of the cell, cancer, birth defects, and hereditary changes in offspring. Damage to the RNA causes impaired protein synthesis, responsible for many types of diseases. The structure of DNA and RNA is illustrated. Note that DNA is double-stranded, known as the double helix. RNA is a single strand of nucleotides.
 |
 |
 |
 |
| |

V. C. Scanlon and T. Sanders, Essentials of
Anatomy and Physiology, 2nd edition. F. A. Davis, 1995. |
|
Adenosine triphosphate (ATP) is the most important high-energy compound. It is a specialized nucleotide, located in the cytoplasm of cells, which serves as a source of cellular energy. ATP contains adenine (amino acid base), ribose (sugar), and three phosphate groups. ATP is created from adenine diphosphate using the energy released during glucose metabolism. One of the phosphates in ATP can later be released along with energy from the broken bond induced by a cellular enzyme.

  
|
|
|
|